You will get information on mouseover the graphic on the right side.
>
OCM (Organizational Change Management) / Schulungen:
Dasselbe, was für die Produktions SOPs gilt, betrifft auch andere Veränderungen im Company – nicht nur ein Software System verändert sich, sondern oft auch Organisationsstrukturen und damit die Rollen der einzelnen Mitarbeiter im Company. Kommunikation ist hier der Schlüssel zur Vorbereitung dieser Veränderung – die Information muss dort ankommen, wo sie benötigt wird – bei Ihren Mitarbeitern. Basierend auf den Blueprintergebnissen werden ein Schulungskonzept, Schulungspläne und korrespondierende Schulungsinhalte entwickelt – damit Ihre Inbetriebnahme von qualifiziertem Personal begleitet wird.
>
Datenmigration:
Migration und die daraus resultierende Datenqualität sind oft die Hauptursache für große Probleme im Tagesgeschäft - schlimmstenfalls die Einstellung des Betriebs nach der Systeminbetriebnahme. Wir schließen die Lücken durch vollständige Dokumentation der Migrationsschritte – von der Definition der Datenanforderungen Ihres SAP Systems bis zur Datenverifikation nach Ankunft der Daten im SAP System. Das schafft Vertrauen in den Migrationsprozess und gibt Sicherheit.
Methodology consulting
SAP implementation is surely one of the biggest challenges even for companies in non-legally regulated environments. Embarking on this kind of project in the pharmaceuticals field can often stretch standard implementation methods and associated quality assurance measures to their limits. When this happens, project costs can be surpassed, timeframes can be exceeded and validation documentation can be inadequate.
Thanks to our years of experience in SAP implementation for pharmaceutical companies, SWQM has developed a corpus of methods that incorporate solutions which traditional methods overlook. We pay particular attention to the minimising of risks associated with your introduction of SAP, and above all validation risks. To us, this is part of the normal project lifecycle, not an extra project task.
Our implementation method comprises the following segments:
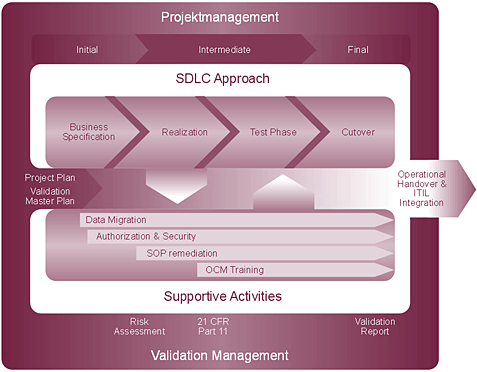
- Project management (according to PMI standard)
- Software development lifecycle
- The business blueprint
At this stage, we lay the foundations for the success of your project. The generation of ideal conditions for the forthcoming realisation phase, clear definitions of the extent of the project (including likely time and cost requirements for development) and a secure basis for DQ (Design Qualification) are the cornerstones of our blueprint phase, during which we compile multi-level process documentation. As a result, we can fully transfer our blueprint structure to the Solution Manager, thereby ensuring support for subsequent phases of the project.


- Realisation
The main tasks of the realisation phase are the configuration of the SAP system according to the blueprint results/specification and the registering of identified developments. The most notable feature of the phase is the integration of inter-phase activities (e.g. data migration) and validation-related activities (e.g. risk analysis). In this area, predefined integration points constitute the unique characteristic of our method: we believe that integrated project performance makes for a successful project.


- Test phase
This phase involves determining a test strategy, defining the dimensions of testing on the basis of the risk analysis and preparing test cases. Our aim is to help customers achieve documented verification of correct system function with trained testers; we are on hand to support you right through to the conclusion of testing.


- Cutover
The cutover starts well before the system goes into productive operation. Cutover planning, the selection of a suitable time, communication within the company and externally, the evaluation of risk and the introduction of appropriate measures, the timely adaptation of production plans for the periods before and after commissioning and, of course, the execution of dry runs: these are just some elements of the cutover phase. We will ensure that you and your employees, customers and partners have every confidence in the cutover process.


- Operational handover/ITIL integration
Validation once and for all is too good to be true! Change requests at various levels, error messages, emergency changes … unexpected problems can arise, especially after the stabilisation phase. However, the integration of ITIL processes within the SAP system environment offers a basis for long-term success (not just in financial terms, but also in terms of compliance with regulatory provisions). Just leave the solutions to us.


- Inter-phase activities
- Data migration
Migration – and the resultant data quality – can often give rise to significant problems in day-to-day business. In the worst case scenario, operations can be shut down after system start-up. We close the gaps by documenting the migration stages in full: from the definition of data requirements for your SAP system to the verification of data transferred to the SAP system. This creates confidence in the migration process and heightens security.


- Authorisation concept
In common with other corporate activities, roles must be assigned and areas of responsibility carefully defined for the SAP system. Our method incorporates the development of a reliable authorisation concept that is sustainable, maintenance-friendly and straightforward. Roles are defined on the basis of the business blueprint and verified under the testing procedure. This ensures that on the day of start-up, each and every employee is authorised to do precisely what they are supposed to be doing – no more, no less.


- Production SOPs (standard operating procedures)
Whenever changes are made to processes or systems within a company, it is virtually certain that SOPs will have to be adapted in the various corporate areas. Pinpointing these SOPs at an early stage, overseeing the adaptation to new processes and thereby ensuring the correspondence of SOPs and system when start-up takes place is another key element of project implementation.


- OCM (organisational change management) and training
The same principle that applies to production SOPs also applies to other changes within a company. It is not only software systems that are modified: changes to organisational structures can also impact on the roles of individual staff members. In such cases, communication is the key to preparing for the change. It is critical that information reaches the people who need it most: your employees. An educational concept, training plans and corresponding content are developed on the basis of the blueprint results. In this way, qualified personnel will be available to support the start-up at your company.


- Special validation activities (risk analysis, 21 CFR Part 11 assessment)
Our methodology is highly adaptable, which means it can be individually tailored to every customer: either in whole (with all of its integral components) or in part, choosing only the specific elements needed for your SAP project. Where the whole method is deployed, the project is shaped to a large extent by the validation master plan and its associated project activities. The method thus supports your validation needs whilst ensuring the overall project can be planned, costed and monitored.


